Molecular modeling of ion channels
(Foto: Anna Weinzinger)
Ion Channels in Health and Disease:
From Mechanistic Understanding to Therapeutic Solutions
Research focus of the Weinzinger group:
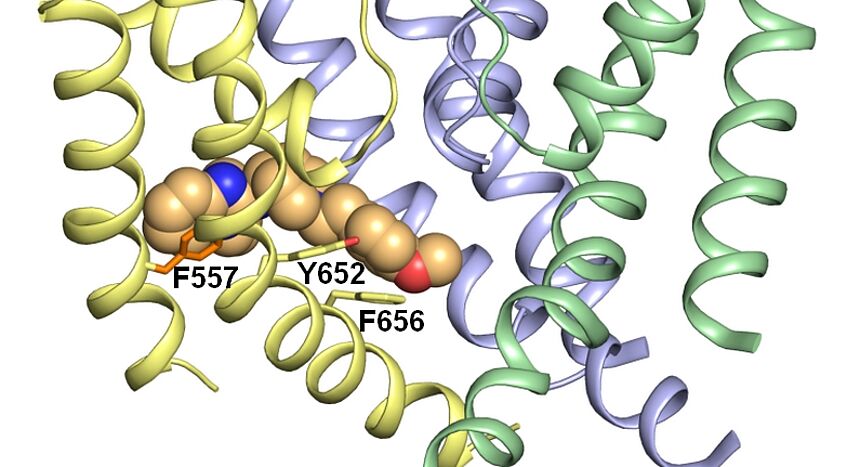
Ion channels are essential for cellular communication, playing a critical role in processes such as nerve signaling, muscle contraction, and hormone secretion. Our research group focuses on the molecular modelling of ion channels to uncover their mechanisms of action, understand their role in disease, and identify novel therapeutic targets. By combining state-of-the-art computational techniques with experimental validation, we aim to advance the field of ion channel research and contribute to drug discovery efforts.
Invention: “Drug screen for rare disease” (Technology ID 2025/05, the University of Vienna has claimed the rights of our invention in 03/2025)

(Foto: Michael Bründl)
News
Van Phuong Nguyen
Room: 2D179
Van Phuong Nguyen was awarded an ERNST MACH Fellowship for his PhD project "Potassium Kv10.1 Channel-Lipid Dysregulation in Diseases".
Publications:
A complete list of publicatons can be found here
Funding:
Potassium Kv10.1 Channel-Lipid Dysregulaton in Diseases
Ernst Mach Grant, ASEA-UNINET, Federal Ministry of Women, Science and Research (BMFWF), Austria´s
Agency for Educaton and Internationalisation, PhD scholarship to Van Phuong NGUYEN
2025 → 2026
Development of allosteric Interactors of GIRK channels, derived from Ethosuximide
India-Austria Science and Technology Cooperation (OeaD) in collaboration with Ass. Prof. Dr. Rambabu
REDDI, www.chemistry.iitkgp.ac.in/professor/rambabu
2025 → 2027
Novel approaches to investigate the regulation of G-protein coupled inward rectifier K+ channels by Gβγ
Austrian Acacemy of Sciences (ÖAW), PhD scholarship to Theres FRIESACHER
2021 → 2024
Prostaglandines – potential therapeutics for DEND syndrome?
Hochschuljubiläumsfonds der Stadt Wien
2020 → 2023
Sulfonylurea to treat Cantu syndrome (CantuTreat)
Austrian Science Fund (FWF), e-rare2
2015 → 2019
hEAG1 channel blockers
Johanna Mahlke geb. Obermann-Stiftung zur Förderung der Krebsforschung an der Universität Wien
2014 → 2015
hERG K+ Channel activators
Hochschuljubiläumsfonds der Stadt Wien
2014 → 2015
Molecular Drug Targets (MolTag)
Austrian Science Fund (FWF)
2010 → 2024
hERG potassium channel
Hochschuljubiläumsfonds der Stadt Wien
2010 → 2011
